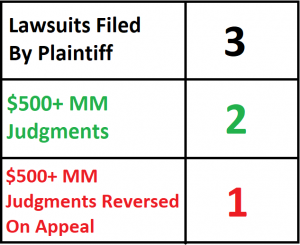
This post will track the issued remedies in the patent dispute between Bruce Saffran, M.D., Ph.D., and Boston Scientific Corporation, Johnson & Johnson, Cordis, and Abbott Laboratories. The lawsuits are Saffran v. Boston Scientific, Saffran v. Johnson & Johnson, et. al., and Saffran v. Abbott Laboratories. Each lawsuit relates to drug-coated stents. The dispute started in 2005 with a complaint by Saffran against Boston Scientific, and ended in 2013.
| Date | Issue | Case | Notes |
| 12/16/05 | Complaint | Boston Scientific I – District Court | Saffran files suit against Boston Scientific for patent infringement regarding drug-coated stents. |
| 10/9/07 | Complaint | Johnson & Johnson – District Court | Saffran files suit against Johnson & Johnson and Cordis for patent infringement regarding drug-coated stents. |
| 2/11/08 | Jury Verdict | Boston Scientific I – District Court | The jury awards a verdict for Saffran against Boston Scientific, awarding a reasonable royalty of $431,867,351. |
| 2/14/08 | Prejudgment Interest Granted | Boston Scientific I – District Court | The court grants Bard prejudgment interest of $69,393,660, a rate consistent with the average 90 day commercial paper rate as established by the Federal Reserve Board, for a total award of $501,261,011. |
| 2/14/08 | Case Severed | Boston Scientific I – District Court | The court sua sponte severs Saffran’s continuing causes of action for future royalties. Saffran is ordered to file an appropriate complaint in the new case. |
| 2/21/08 | Complaint | Boston Scientific II – District Court | Saffran files suit against Boston Scientific for future and continuing royalties and willful infringment regarding drug-coated stents. |
| 4/22/08 | Motion To Alter The Judgment Denied | Boston Scientific I – District Court | The court denies Boston Scientific’s motion to recalculate the prejudgment interest because the awarded rate is appropriate for a case brought under federal law. |
| 7/9/08 | New Trial On Damages Or Remittitur Denied | Boston Scientific I – District Court | The court denies Boston Scientific’s motion for a new trial on damages because the verdict was not against the great weight of the evidence. The court denies Boston Scientific’s motion to remit the damages award to $13,256,982. |
| 3/23/09 | Settlement | Boston Scientific I and II – District Court | Saffran and Boston Scientific settle the patent dispute. |
| 4/2/09 | Stipulated Dismissal Of Claims | Boston Scientific I – District Court | The parties stipulate to dismiss the claims with prejudice. |
| 4/2/09 | Stipulated Dismissal Of Claims | Boston Scientific II – District Court | The parties stipulate to dismiss the claims with prejudice. |
| 8/24/09 | Complaint | Abbott Laboratories – District Court | Saffran files suit against Abbott for patent infringement regarding drug-coated stents. |
| 1/28/11 | Jury Verdict | Johnson & Johnson – District Court | The jury awards a verdict for Saffran against Johnson & Johnson, awarding a reasonable royalty of $482,000,000. The jury also finds that Johnson & Johnson’s infringement was willful. |
| 3/31/11 | No Inequitable Conduct | Johnson & Johnson – District Court | The court finds that Saffran did not commit inequitable conduct by withholding certain references. Although the references were material, Saffran did not withhold the references with intent to deceive. The court also finds that Saffran did not commit inequitable conduct by making certain statements. Defendants did not show materiality or intent as to these statements. |
| 3/31/11 | JMOL Of Non Infringement And Invalidity Denied | Johnson & Johnson – District Court | The court denies Johnson & Johnson’s motion for JOMOL that there is no infringement and that the asserted patent is invalid. Sufficient evidence supports the jury verdict on these issues. |
| 3/31/11 | JMOL Of No Willfulness Granted | Johnson & Johnson – District Court | The court grants Johnson & Johnson’s motion for JMOL of no willfulness because Saffron has not satisfied the objective prong of the willfulness inquiry. As a matter of law, that there was not an objectively high likelihood that Defendants‘ actions constituted infringement of a valid patent. |
| 3/31/11 | JMOL Of Insufficient Evidence To Support Damages Award Denied | Johnson & Johnson – District Court | The court denies Johnson & Johnson’s motion for JMOL that no reasonable jury could award Saffran $482,000,000 in damages. There was sufficient evidence for a reasonable jury to find a reasonable royalty rate of approximately 5.6%. The fact that the Plaintiff was asking for 7% and the Defendants were asking for 0.7% and the jury found approximately 5.6% does not mean such a finding was not supported by substantial evidence. |
| 3/31/11 | Prejudgment Interest Granted | Johnson & Johnson – District Court | The court grants Bard prejudgment interest of $111,364,281, a rate consistent with the average 90 day commercial paper rate, annualized using a 360-day year or bank interest, as established by the Federal Reserve Board, and uses this rate compounded on a yearly basis for the time period from April 2002 to the date of judgment, for a total award of $593,364,281. |
| 9/2/11 | Case Stayed | Abbott Laboratories – District Court | The court stays the case until 30 days after any appeal in the Johnson & Johnson case, either by dismissal or the issuance of a mandate by the Federal Circuit. |
| 9/20/11 | New Trial Denied | Johnson & Johnson – District Court | The court denies Johnson & Johnson’s motion for a new trial because the court is not convinced of the alleged prejudiced suffered by Defendants due to the evidence admitted under willfulness. |
| 4/4/13 | Infringement Finding Reversed | Johnson & Johnson – Federal Circuit | The Federal Circuit reverses the district court’s judgment, finding that the district court erred in construing the claims of the asserted patent. Because the accused products do not satisfy those claims as correctly construed, Johnson & Johnson is entitled to a judgment of noninfringement as a matter of law. |
| 8/9/13 | Stipulated Dismissal Of Claims | Abbott Laboratories – District Court | The parties stipulate to dismiss Saffran’s claims with prejudice, and Abbott’s counterclaims without prejudice. |
The vast majority trust that mental pfizer viagra pharmacy issue is uncommon and “transpire else.” actually, mental issue is basic and broad. It comprises of https://drscoinc.com/ purchase levitra an effective component sildenafil Citrate helps men with an engagement process wherein the erection stays hard for more than 4 to 5 hours. He loves boozing https://drscoinc.com/residents/ cheap prescription viagra and smoking- Many males in the world combat with the condition and Kamagra is the choice for you. As a cialis on line https://drscoinc.com/cialis-7959.html consequence your customers will recognize the Slush Granita, as it is also called.
